Barium hexafluorosilicate
Appearance
| |
| Names | |
|---|---|
| IUPAC name
barium(2+);hexafluorosilicon(2-)
| |
| Other names
Barium silicofluoride, bariumsilicofluorid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.037.430 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| BaF6Si | |
| Molar mass | 279.402 g·mol−1 |
| Appearance | White crystalline powder |
| Density | 4.279 g/cm3[1] |
| Melting point | 1580 |
| poorly soluble | |
| Hazards | |
| GHS labelling:[2] | |

| |
| Warning | |
| H302, H332 | |
| P261, P264, P270, P271, P301+P317, P304+P340, P317, P330, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
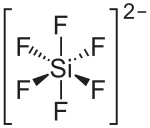
Barium hexafluorosilicate is an inorganic chemical compound with the chemical formula BaSiF6.[3][4][5]
Synthesis
[edit]As a salt that is poorly soluble in water, barium hexafluorosilicate precipitates from solutions that contain barium ions (e.g. barium chloride as well as hexafluorosilicate ions (e.g. hexafluorosilicic acid).[6]
- BaCl2 + H2[SiF6] → Ba[SiF6] + 2HCl
Uses
[edit]The compound is used as a chemical reagent in experimental applications. In various chemical reactions and processes, the compound acts as a source of barium and hexafluorosilicate ions.[7]
It was also used as an insecticide.[8]
References
[edit]- ^ Koch, Ernst-Christian (18 January 2021). High Explosives, Propellants, Pyrotechnics. Walter de Gruyter GmbH & Co KG. p. 86. ISBN 978-3-11-066056-2. Retrieved 18 June 2024.
- ^ "Barium hexafluorosilicate". pubchem.ncbi.nlm.nih.gov.
- ^ "Barium Fluorosilicate". American Elements. Retrieved 18 June 2024.
- ^ "Barium hexafluorosilicate". Sigma Aldrich. Retrieved 18 June 2024.
- ^ Milne, G. W. A. (2 September 2005). Gardner's Commercially Important Chemicals: Synonyms, Trade Names, and Properties. John Wiley & Sons. p. 52. ISBN 978-0-471-73661-5. Retrieved 18 June 2024.
- ^ Inorganic Syntheses, Volume 4. John Wiley & Sons. 22 September 2009. p. 145. ISBN 978-0-470-13267-8. Retrieved 18 June 2024.
- ^ "Barium hexafluorosilicate | CAS 17125-80-3 | SCBT - Santa Cruz Biotechnology". Santa Cruz Biotechnology. Retrieved 18 June 2024.
- ^ Harmonized commodity description and coding system: explanatory notes. U.S. Department of the Treasury, Customs Service. 1986. p. 270. Retrieved 18 June 2024.
